Btk Kinase inhibitor: Mechanism, Application And Toxicity Studies
General Description
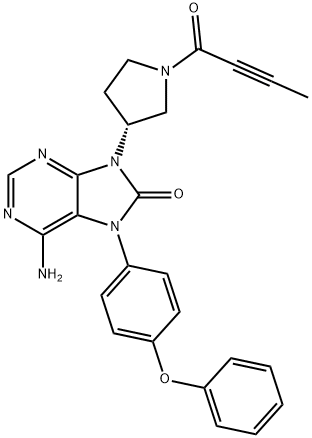
Btk Kinase inhibitor(Velexbru®) with CAS: 1351636-18-4, is highly potent and selective orally administered, small molecule, and also named tirabrutinib, Brutons tyrosine kinase (BTK) inhibitor being developed by Ono pharmaceutical and its licensee Gilead Sciences for the treatment of autoimmune disorders and haematological malignancies. Btk Kinase inhibitor irreversibly and covalently binds to BTK in B cells and inhibits aberrant B cell receptor signalling in B cell-related cancers and autoimmune diseases. In March 2020, oral Btk Kinase inhibitor was approved in Japan for the treatment of recurrent or refractory primary central nervous system lymphoma. Btk Kinase inhibitor is also under regulatory review in Japan for the treatment of Waldenströms macroglobulinemia and lymphoplasmacytic lymphoma. Clinical development is underway in the USA, Europe and Japan for autoimmune disorders, chronic lymphocytic leukaemia, B cell lymphoma, Sjogrens syndrome, pemphigus and rheumatoid arthritis.[1]

Figure 1 Btk Kinase inhibitor Powder
Mechanism
The selective inhibition of cell growth by Btk Kinase inhibitor was due to blocking of BTK-mediated signaling through AKT and cellular protein kinase D. It can inhibit autophosphorylation of the BTK at the Tyr223 position through the ERK, AKT and PKD signaling pathways.[2]
Application
The treatment with Btk Kinase inhibitor in combination with chemotherapy results in a synergistic effect, inducing apoptosis and is more effective compared with respective monotherapies. Btk Kinase inhibitor is currently being developed in a Phase I clinical trial for the treatment of B-cell malignancies.[3]
Btk Kinase inhibitor irreversibly and covalently binds to BTK in B cells and has demonstrated effective in vitro cytotoxicity in many types of B-cell malignancies and in vivo antitumor activity in mouse models.[4]In an ABC-DLBCL cell line (TMD-8) xenograft model, the effects of Btk Kinase inhibitor on gene transcription in vivo were analyzed,the results indicated that Btk Kinase inhibitor affects a core set of genes that contain nine down-regulated and eight up-regulated genes in a dose-dependent manner. Among these, CXCL-10 is the most down-regulated gene by Btk Kinase inhibitor and is involved in the pathological processes of human disorders, such as infectious diseases and inflammatory and autoimmune diseases as well as cancer. profound anti-proliferative activity of Btk Kinase inhibitor by inhibiting BTK in the TMD-8 model. The first-in-human phase I study of Btk Kinase inhibitor was on relapsed/refractory B cell malignancies (NCT01659255).Btk Kinase inhibitor was found to be well tolerated, with no dose limiting toxicities (DLTs). The pharmacokinetics of Btk Kinase inhibitor reflects rapid absorption and elimination, a half-life of 6 hours, a dose dependent increase in exposure with no accumulation of Btk Kinase inhibitor exposure and low inter- or intra-patient variability; with Btk occupancy in peripheral blood (as measured by phosphorylated Btk) being maintained for at least 24 hours across all dose levels.
In the kinomescan study, Btk Kinase inhibitor was found to have significantly weaker activity on TEC kinase. Therefore, Btk Kinase inhibitor has a favorable safety profile along with preliminary efficacy in patients with relapsed/refractory B cell malignancies. Combination of idelalisib and Btk Kinase inhibitor synergistically inhibited the growth of a subset of DLBCL and MCL cell lines. This combination led to more significant growth inhibition of the A20 mutant TMD8 cells than single agent idelalisib. This suggested that the combination therapy may overcome some mechanisms of resistance in the BTK signaling pathway. In addition, Jones et al. investigated the potential activity of combinations of the B cell receptor pathway inhibitors, entospletinib, ONO/GS4059, and idelalisib, with the BCL2 inhibitor ABT-199 in primary CLL cells. Results showed that their combination synergistically increased the apoptosis in these primary CLL cells and achieved the maximal levels of apoptosis.[5]
Toxicity
Adverse effects of the Btk Kinase inhibitor that occurred in some patients were rash, vomiting, neutropenia, arthralgia, and malaise, and drug-related Grade 3–4 Adverse effects were neutropenia, leukopenia, anemia, hypophosphatemia, PT-INR increased, pneumonitis, and acute myeloid leukemia. in prior Japanese studies, rash, hematologic adverse effects, erythema multiforme, and constipation were frequent adverse effects in a Phase I/II study of some patients with PCNSL, and rash, hematologic adverse effects, and stomatitis were the most common adverse effects in a Phase II study of some patients with WM. In international studies, other frequently reported adverse effects included hematologic adverse effects, diarrhea, petechiae, rash, nasopharyngitis, upper respiratory tract infection, fatigue, cough, and arthralgia.Infections were also evaluated as adverse effects of specific interest, and included cystitis, nasopharyngitis, pharyngitis, and sinusitis. There was one Grade ≥ 3 infection-related adverse effect(pneumonia bacterial). Although the infection-related adverse effects were manageable in most patients with prophylactic use or treatment with antibacterial agents, some caution may be necessary, especially in patients with serious infections.[6]
Reference
[1]Dhillon S. Tirabrutinib: first approval[J]. Drugs, 2020, 80(8): 835-840.
[2]Wu J, Zhang M, Liu D. Bruton tyrosine kinase inhibitor Btk Kinase inhibitor: from bench to bedside[J]. Oncotarget, 2017, 8(4): 7201.
[3]Tomoko Yasuhiro,Toshio Yoshizawa, PhD,Joseph TP Birkett, PhD,Kazuhito Kawabata, PhD.Btk Kinase inhibitor, A Novel Bruton’s Tyrosine Kinase (Btk) Inhibitor: Synergistic Activity In Combination With Chemotherapy In a ABC-DLBCL Cell Line.[J].Blood,2013,122 (21): 5151.
[4]Munakata W, Tobinai K. Tirabrutinib hydrochloride for B-cell lymphomas[J]. Drugs of Today (Barcelona, Spain: 1998), 2021, 57(4): 277-289.
[5]Wu J, Liu C, Tsui S T, et al. Second-generation inhibitors of Bruton tyrosine kinase[J]. Journal of hematology oncology, 2016, 9(1): 1-7.
[6]Munakata W, Ando K, Yokoyama M, et al. Long-term safety profile of tirabrutinib: final results of a Japanese Phase I study in patients with relapsed or refractory B-cell malignancies[J]. International Journal of Hematology, 2022: 1-10.
Related articles And Qustion
Lastest Price from Tirabrutinib manufacturers

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10kg

US $3.00/KG2022-09-01
- CAS:
- 1351636-18-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG